Join us in the Fight for Cancer Patients:
The Right Drug for the Right Patient Right Now!
Access to Comprehensive Molecular Profiling for All Patients
Comprehensive molecular profiling, or biomarker testing, is the key to unlocking precision medicine. This crucial testing enables patients and their doctors to precisely target cancer by identifying therapies with potentially greater benefit, while ruling out drugs that are less likely to work and thus avoiding unnecessary toxicities and costs. Due to what we believe to be outdated medical coverage policies, however, many insurance providers limit coverage and access to comprehensive biomarker testing, which may increase cancer care disparities and negatively impact patient care.
Driven by an unwavering devotion to impact all cancer patients, Caris’ comprehensive molecular profiling leaves no stone unturned to help guide treatment decision and ongoing cancer care management. Propelled by a decade of innovation and relentless commitment to help build a better world, we are not waiting for change. We are the change – and it’s happening now.
Join us in the fight to improve access and coverage for cancer patients.
We are working with lawmakers to improve access and insurance coverage for biomarker testing, as well as working with insurance providers to update medical coverage policies to ensure alignment with industry guidelines that reflect current standards of care and clinical practice for the benefit of cancer patients.
Comprehensive Molecular Profiling is Standard of Care
Significant evidence supporting gene profiling using sequencing panels that cover more than 50 genes continues to be published, including from the National Comprehensive Cancer Network® (NCCN), the American Society of Clinical Oncology (ASCO), and others:
- NCCN Guidelines® NSCLC (2023)1
- Testing should be performed via a broad, panel-based approach
- ASCO Provisional Clinical Opinion (2022)2
- Genomic testing should be performed for patients with metastatic or advanced solid tumors
- Multigene panel–based testing is defined as an NGS test with at least 50 genes
- Large multigene panel-based testing should be performed to assess MSI, TMB and gLOH
- Contemporary Clinical Practice Patterns Study (2022)3
- Comprehensive NGS-based panels reduce the likelihood of missing targeted therapy options compared to smaller, non-NGS panels
Comprehensive Molecular Profiling Provides Value Across the Patient Journey
A comprehensive profiling approach enables oncologists to select the right therapy while avoiding costly non-beneficial therapies. In fact, more than 20 states have enacted, passed or introduced comprehensive biomarker testing bills to expand coverage and patient access in the U.S.4
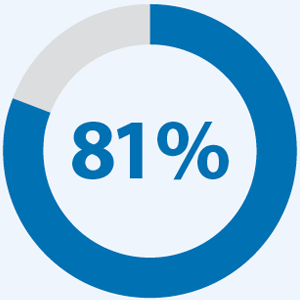
of alterations are missed by small hotspot panels.5
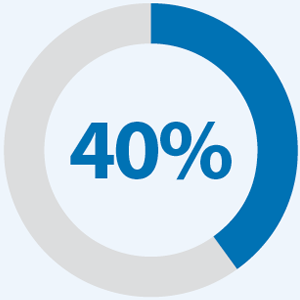
of patients tested with non-NGS methods had a missed targeted therapy versus 3% of patients who underwent CMP.3
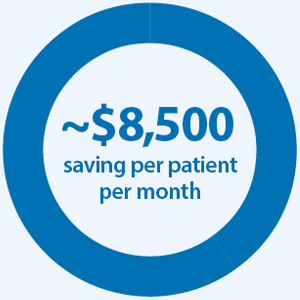
in overall total cost of care due to more optimal treatment in patients with lung cancer who received broad versus narrow panel testing.6
Join the list of healthcare providers and patients that support comprehensive molecular profiling.
"*" indicates required fields
Caris Molecular Profiling
Comprehensive tumor profiling from Caris assesses DNA and RNA via next-generation sequencing to reveal a molecular blueprint to guide more precise and individualized treatment decisions.- Accreditation: CAP, CLIA, ISO
- Comprehensive Testing across DNA and RNA
- Whole Exome Sequencing: SNVs, InDels, CNAs, MSI, TMB, gLOH, HRD, HLA Genotyping
- Whole Transcriptome Sequencing: Fusions, Variant Transcripts
- AI-Based Predictive Signatures: Caris GPSai™ (Tumor Type ID), Caris FOLFIRSTai™ (response predictor for mCRC)
Insurance Providers in the Caris Profiling Network
Caris is proud to partner with more than 90 insurance providers, covering more than 265 million patients, who recognize the clinical value of comprehensive molecular profiling for both physicians and patients.Fighting Cancer Smarter
Knowing what cancer looks like at the molecular level can lead to better treatment options.
Patient Videos
Check out our Video Library to learn more about Caris molecular profiling.
- National Comprehensive Cancer Network (NCCN) Guidelines: Non-Small Cell Lung Cancer. Version 3.2023. Retrieved from NCCN Guidelines: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Chakravarty D, et al. Somatic Genomic Testing in Patients With Metastatic or Advanced Cancer: ASCO Provisional Clinical Opinion. Journal of Clinical Oncology 40, 1231-1258. doi:10.1200/jco.21.02767
- Paz-Ares L, et al. (2022) Genomic testing among patients with newly diagnosed advanced Non-Small Cell Lung Cancer in the United States: A contemporary clinical practice patterns study. Lung Cancer 167, 41-48. doi: 10.1016/j.lungcan.2022.01.021
- Access to Biomarker Testing. American Cancer Society. Oct 2023. www.fightcancer.org/what-we-do/access-biomarker-testing
- Zehir A, et al. (2017) Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature Medicine 23, 703-713. doi:10.1038/nm.4333
- Brito RA, et al. (2020) Total cost of lung cancer care associated with broad panel versus narrow panel sequencing. Journal of Clinical Oncology 38, 7077-7077. doi:10.1200/jco.2020.38.15_suppl.7077