Homologous Recombination Deficiency
Caris Life Sciences identifies tumors that exhibit Homologous Recombination Deficiency (HRD), an important biomarker for advanced ovarian cancer and PARP inhibitor (PARPi) therapy. Detection of altered HRD genes such as BRCA may help identify this molecular dysfunction, but other non-BRCA genes or epigenetic factors may also be involved in HRD. It is important to verify HRD-related genomic scarring, such as Genomic Loss of Heterozygosity (gLOH) and Large-scale State Transitions (LST), in order to best inform treatment.
- HRD-positive tumors may be sensitized to PARPi therapy.
- HRD is present in nearly half of all ovarian cancer tumors.1
- HRD is often assessed solely on BRCA alterations, however BRCA alterations only account for half of HRD+ cases.2
- Non-BRCA causes of HRD include other gene alterations and epigenetic factors.
- Identifying HRD-positive tumors based on genomic scarring in addition to BRCA alterations results in a more complete assessment of HRD.

Caris HRD Status*
Reported for epithelial ovarian cancer cases (tissue profiling) at no additional cost or specimen requirements.
*Not available in all locations.
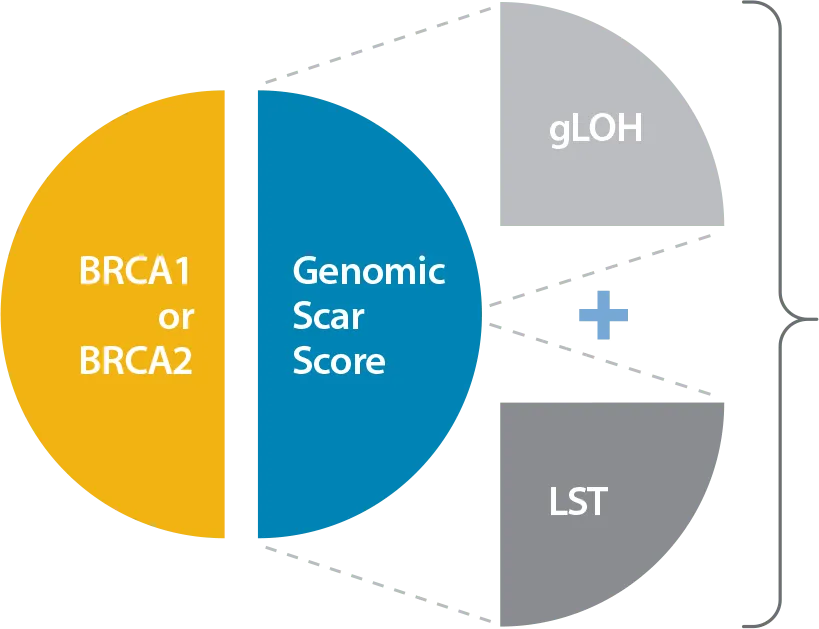
Caris reports HRD status based on BRCA1 or BRCA2 mutations and a Genomic Scar Score (GSS), comprised of genomic Loss of Heterozygosity (gLOH) plus Large-scale State Transitions (LST):
HRD-positive:
Either BRCA mutation detected or GSS = HIGH
HRD-negative:
BRCA mutation not detected and GSS = LOW
HRD and PARPi Therapy
Homologous Recombination (HR) is a cellular process for maintaining chromosomal integrity. Alterations in one or several HR genes may result in HR Deficiency (HRD). Dysfunctional HR proteins cannot repair double-stranded DNA breaks, and other repair pathways such as poly-ADP-ribose-polymerase (PARP) or non-homologous end joining (NHEJ) cannot compensate, resulting in genome-wide errors and tumorigenesis.3
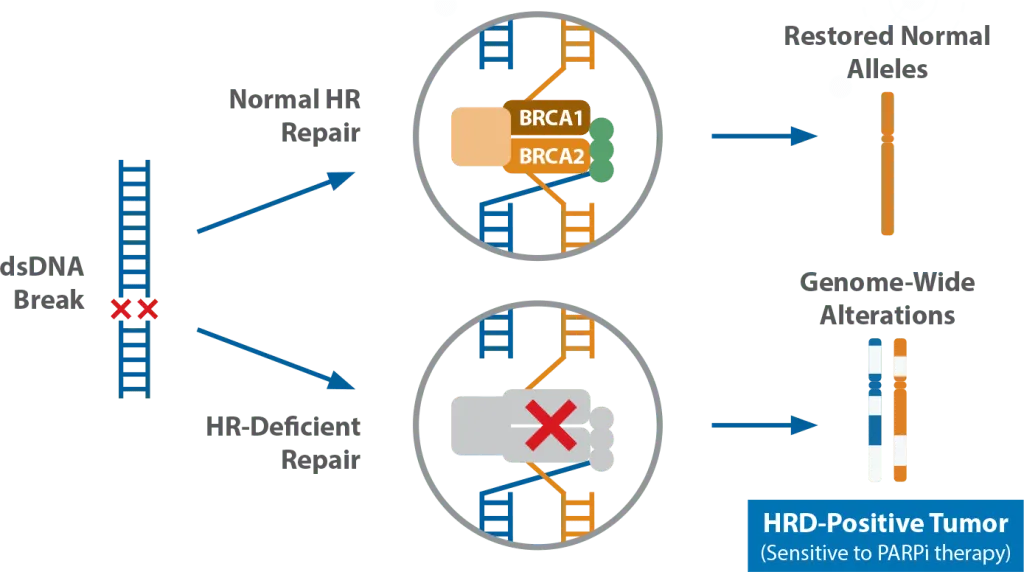
Figure 1. Homologous Repair Deficiency (HRD) is the result of genetic alteration(s) in HR genes (eg, BRCA1, BRCA2 and others) or epigenetic factors. Dysfunctional HR genes result in genome-wide errors and tumorigenesis.
Easy to Interpret HRD Results
Results with Therapy Associations
| BIOMARKER | METHOD | ANALYTE | RESULT | THERAPY ASSOCIATIONS | BIOMARKER LEVEL* | |
|---|---|---|---|---|---|---|
| HRD** | Seq | DNA-Tumor | Positive | BENEFIT | niraparib, olaparib, rucanparib | Level 2 |
| BRCA1 | Seq | DNA-Tumor | Pathogenic Variant Exon 10 | p.l650fs | |||
* Biomarker reporting classification: Level 1 – Companion Diagnostics (CDx); Level 2 – Strong evidence of clinical significance or is endorsed by standard clinical guidelines; Level 3 – Potential clinical significance. Bolded benefit therapies, if present, highlight the most clinically significant findings.
** HRD – homologous recombination deficiency status. A positive result is indicative of a pathogenic or likely pathogenic variant(s) in BRCA1 and/or BRCA2 or high Genomic Scar Score (LOH + LST) which consists of genomic Loss of Heterozygozity (gLOH) + Large-scale State Transitions (LST).
1. Zhang H, Liu T, Zhang Z, et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer. Cell. 2016;166:755-765.
2. Sugino K, et al. Sci Rep. 2019;9[1]:17808; Pennington KP, et al. Clin Cancer Res. 2014;20[3]:764–775).
3. Ngoi NYL, Tan DSP. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: do we need it? ESMO Open. 2021 Jun;6(3):100144.
Discover
More
Caris molecular profiling includes microsatellite instability (MSI) testing via next-generation sequencing (NGS).
The Human Leukocyte Antigen system is largely responsible for regulating the immune system.
Speak to an Expert
"*" indicates required fields


