Microsatellite Instability (MSI)
Caris molecular profiling includes microsatellite instability (MSI) testing via next-generation sequencing (NGS). MSI is caused by failure of the DNA mismatch repair (MMR) system. High levels of MSI correlate to an increased neoantigen burden, which may make the tumor more sensitive to immunotherapy. MSI status is reported on pages one and two of the Caris Report, as well as in the NGS section in the Appendix. Caris comprehensive profiling adheres to CAP Guidelines for MSI and MMR testing.
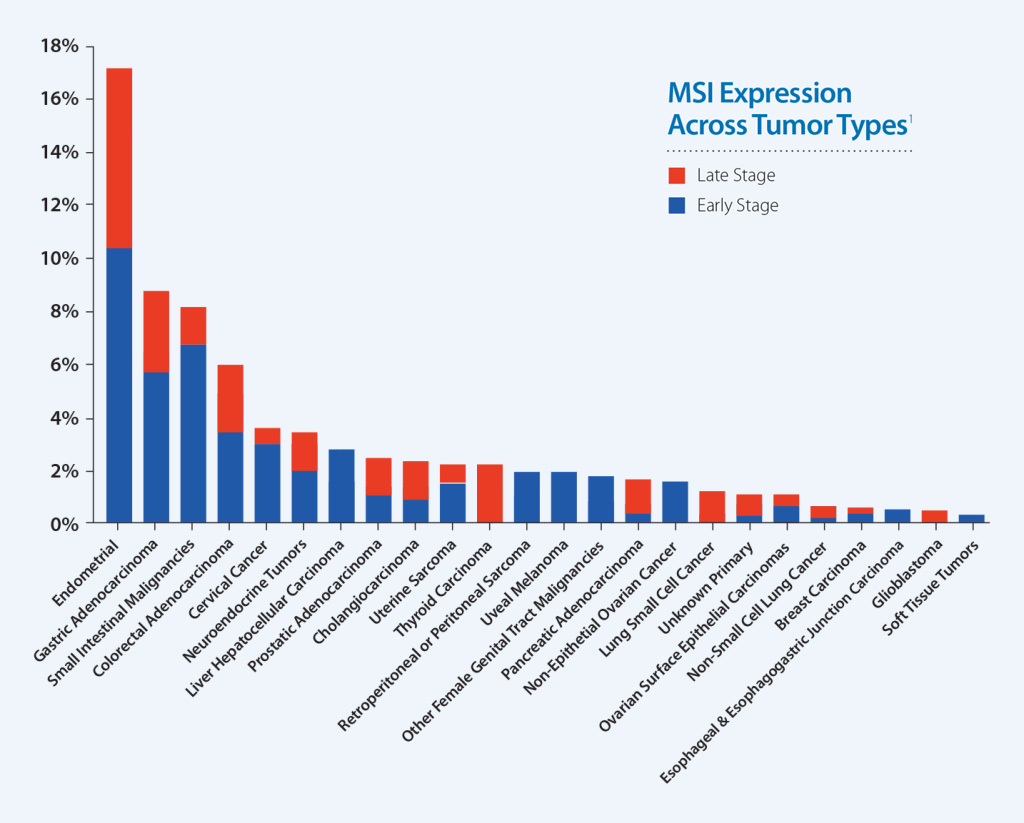
MSI-High Status Across Caris Molecular Profiling Cases
Earlier studies have associated MSI-High status with benefit to immunotherapy in metastatic colorectal cancer. Additional data show that MSI is a useful indicator for predicting response to pembrolizumab in any solid tumor type.1
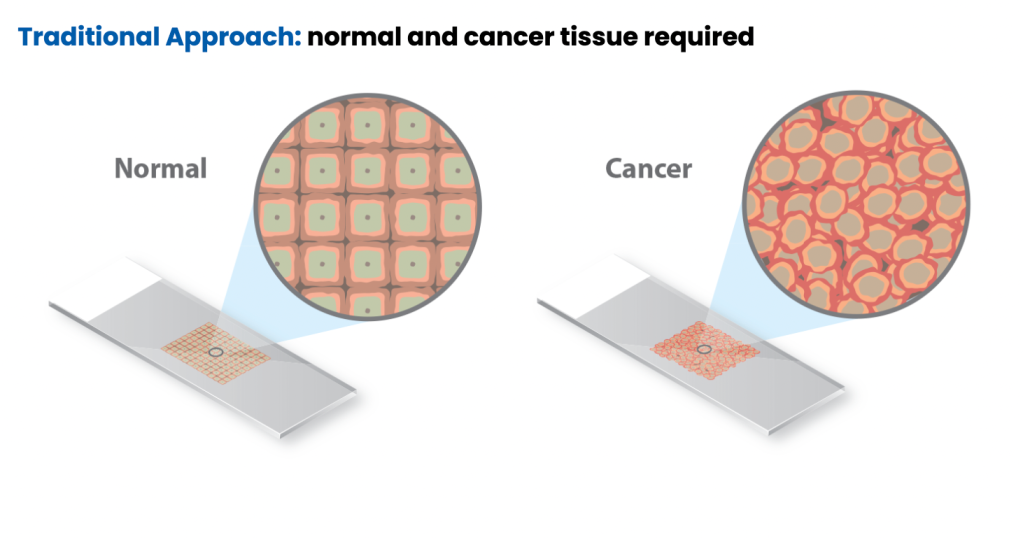
Traditionally, MSI is detected through polymerase chain reaction (PCR) by fragment analysis (FA) of five conserved satellite regions and comparing cancer tissue to normal tissue to identify differences in tandem repeats.3-4 To validate MSI testing via NGS, Caris evaluated more than 7,000 target microsatellite loci and compared the results from PCR for 2,189 cases across 26 different tumor types. This data was published in Cancer Medicine and demonstrated that MSI testing with Caris’ NGS platform is highly concordant with the traditional standard method of PCR-FA and is a more efficient and cost-effective approach to identifying patient candidates for immunotherapy.2
| Concordance Data: PCR vs NGS2 | ||||
|---|---|---|---|---|
| Lineage | Sensitivity | Specificity | PPV | NPV |
| All | 95.8% | 99.4% | 94.5% | 99.2% |
| CRC | 100.0% | 99.9% | 98.7% | 98.7% |
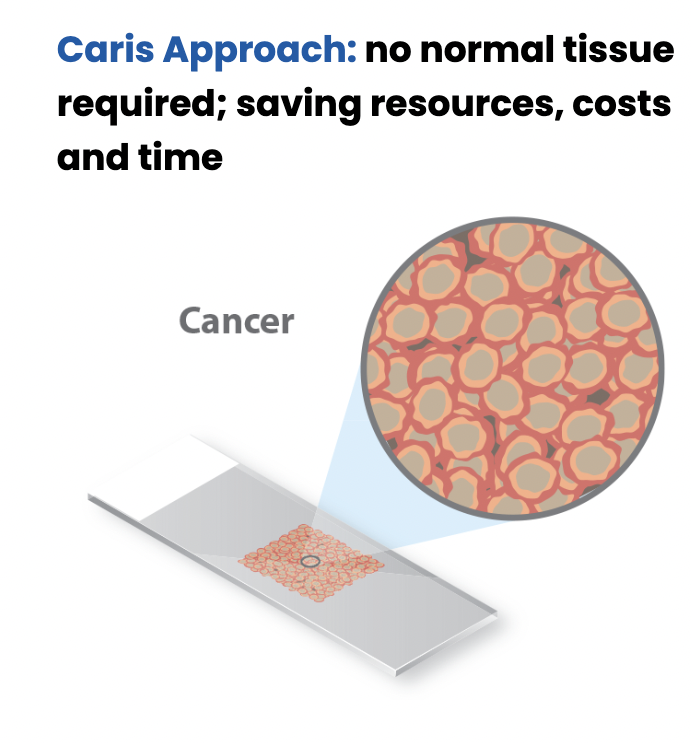
MSI testing is provided at no additional cost and no increase in specimen requirements or turnaround time when Caris molecular profiling is ordered. The results can be found in the Genomic Signatures section of the Caris molecular profiling report, alongside Genomic LOH (gLOH) and Tumor Mutational Burden (TMB) results.
- T. Le, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. Published Online 8 June 2017. DOI: 10.1126/science.aan6733.
- Vanderwalde, A., Spetzler, D., Xiao, N., Gatalica, Z. and Marshall, J. (2018), Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. doi:10.1002/cam4.1372
- de la Chapelle, A., and H. Hampel. 2010. Clinical relevance of microsatellite instability in colorectal cancer. J. Clin. Oncol. 28:3380–3387.
- Zhang, L. 2008. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J. Mol. Diagn. 10:301–307.
Discover
More
Tumor Mutational Burden is a pan-tumor biomarker that indicates a cancer may respond more effectively to immunotherapies.
Genomic Loss of Heterozygosity or genomic instability is often related to defective homologous recombination repair mechanisms.
Speak to an Expert
"*" indicates required fields
