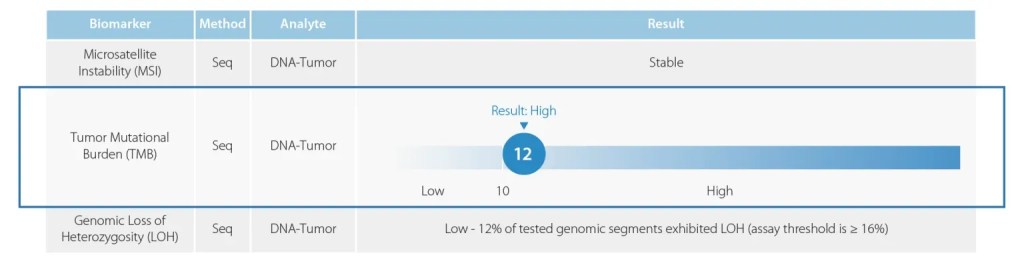
Tumor Mutational Burden
Tumor Mutational Burden (TMB) by Whole Exome Sequencing measures the total number of mutations that result in altered proteins (neo-antigens), a pan-tumor biomarker that indicates a cancer may respond more effectively to immunotherapies. The Caris TMB assay quantifies the number of non-synonymous, somatic mutations identified per megabase (Mb) of the genome coding area of DNA (1 megabase is 1,000,000 DNA basepairs) and reports results as Low or High.
Non-synonymous mutations are changes in DNA that result in amino acid changes in the protein.1,2 The new protein changes result in new shapes (neo-antigens) that are considered to be foreign to the immune system.1,3 Immune checkpoint inhibitors are able to stimulate and allow the immune system to detect these neo-antigens and destroy the tumor.2 Germline (inherited) mutations are not included in Tumor Mutational Burden because the immune system has a higher likelihood of recognizing these alterations as normal.4
TMB has emerged as an important biomarker when considering immunotherapy in solid tumors. The Caris approach to TMB high is based on the results of the keynote trial and the FDA approval of pembrolizumab (KEYTRUDA®) for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options.
Caris has collaborated with the Friends of Cancer Research TMB Harmonization Project to systematically characterize and standardize Tumor Mutational Burden testing and reporting to a common industry standard.6 Based on this collective work and exciting KEYNOTE-158 result and drug approval, Caris has updated the TMB high/low threshold to reflect greater than or equal to 10 mutations per megabase across all solid tumors, aligning the testing results to pembrolizumab for TMB-H cases.5
Caris Molecular Profiling TMB-H Cutoff Aligned Across All Solid Tumors
- Snyder A. N Engl J Med. 2014; 371:2189-2199. doi:10.1056/ NEJMoa1406498
- Le DT. N Engl J Med. 2015;372:2509-2520. doi:10.1056/NEJMoa1500596
- Rosenberg JE. The Lancet. 2016; 387(10031):1909-1920. doi:10.1016/S0140-6736(16)00561-4.
- Stewart TJ. Oncogene. 2008;27:5894-5903. doi:10.1038/onc.2008.268
- U.S. Food and Drug Administration. (2020, June 16). FDA approves pembrolizumab for adults and children with TMB-H solid tumors [Press release].
- Stenzinger, A. Genes Chromosomes Cancer. 2019; 58: 578– 588. https://doi.org/10.1002/gcc.22733
Discover
More
Caris molecular profiling includes microsatellite instability (MSI) testing via next-generation sequencing (NGS).
Genomic Loss of Heterozygosity or genomic instability is often related to defective homologous recombination repair mechanisms.
