Comprehensive Molecular Profiling
The Leading Innovator in Precision Medicine
Caris has been the leading innovator in molecular profiling since we were first founded in 2008. With the promise of precision medicine becoming a reality, broad molecular profiling has become standard of care for many cancer types – and is required for certain therapies. More than ever, oncologists need a trusted profiling partner to provide reliable, high-quality molecular profiling information to guide precise and individualized treatment decisions. Our comprehensive molecular profiling approach precisely analyzes DNA, RNA, and protein biomarkers, revealing the highest quality molecular blueprint for evidence-based selection of the most appropriate cancer therapy. In addition to comprehensive molecular profiling, Caris has innovated industry-leading artificial intelligence algorithms that predict patient responses to standard treatments, such as immunotherapy or chemotherapy, based on their personalized molecular profile. Caris is the original, most experienced comprehensive molecular profiling laboratory, and we have optimized our systems to provide industry-leading reports, service and turnaround time. Caris molecular profiling helps oncologists:- Navigate among therapies with potential benefit
- Identify therapies that may not have been considered
- Determine drugs with potential lack of benefit (avoiding unnecessary toxicities and costs)
- Match patients to clinical trials
The Most Experienced Comprehensive Molecular Profiling Provider
NEXT-GENERATION SEQUENCING (NGS) AND PROTEIN ANALYSIS EXPERTISE
- Whole Exome Sequencing (WES) from DNA of all 22,000 genes
- Whole Transcriptome Sequencing (WTS) from RNA of all 22,000 genes
- Genomic Signatures (HRD, gLOH, MSI, TMB)
- HLA Genotyping
- Pyrosequencing for methylation/epigenetics analysis
- Protein Analysis (IHC, CISH, FISH)
IMMUNOTHERAPY DIAGNOSTICS EXPERTISE
- PD-L1 by IHC, including CPS, TPS, TC and IC scoring methods
- Mismatch Repair (MMR) proteins by IHC: MLH1, MSH2, MSH6, PMS2
- Microsatellite Instability (MSI) by WES
- Tumor Mutational Burden (TMB) by WES
RIGOROUS QUALITY STANDARDS
- CAP, CLIA, NYSDOH, ISO15189 accredited
- 66,000-square-foot clinical laboratory in Phoenix, AZ
- 38,000 square-foot R&D facility in Phoenix, AZ
- 115,000-square-foot blood-based laboratory in Irving, Texas
- Staffed by: bioinformaticians, oncologists, molecular geneticists, pathologists and PhD scientists
LIMITED TISSUE CAPABILITIES
- Tumor enrichment via microdissection
- Multiple reflex options to alternative technologies/methods
RAPID TURNAROUND TIME
- 10-14 calendar days
Caris Molecular Profiling Menu by Region
International & US
New York
European Union (EU)
Reading Your Molecular Profiling Reports
Caris molecular profiling performs thorough molecular testing on DNA, RNA and proteins to identify the biomarkers driving a patient’s tumor, and to compare the biomarkers with data from clinical studies around the world. Here’s how the process works:
Sample sent to Caris
The ordering clinician collects and sends a tissue sample to Caris
Biomarker Testing
Comprehensive testing on DNA, RNA and proteins is performed at our state-of-the-art, 66,000 sq. ft. laboratory
Bioinformatics Performed
Team interprets the data using thousands of relevant clinical papers to match your tumor’s biomarkers to treatment options
Report Generated
Results are returned in approximately 10-14 days in an online and easy-to-understand format
Physician Reviews Report
Profiling reveals the molecular blueprint to help guide precise treatment decisions
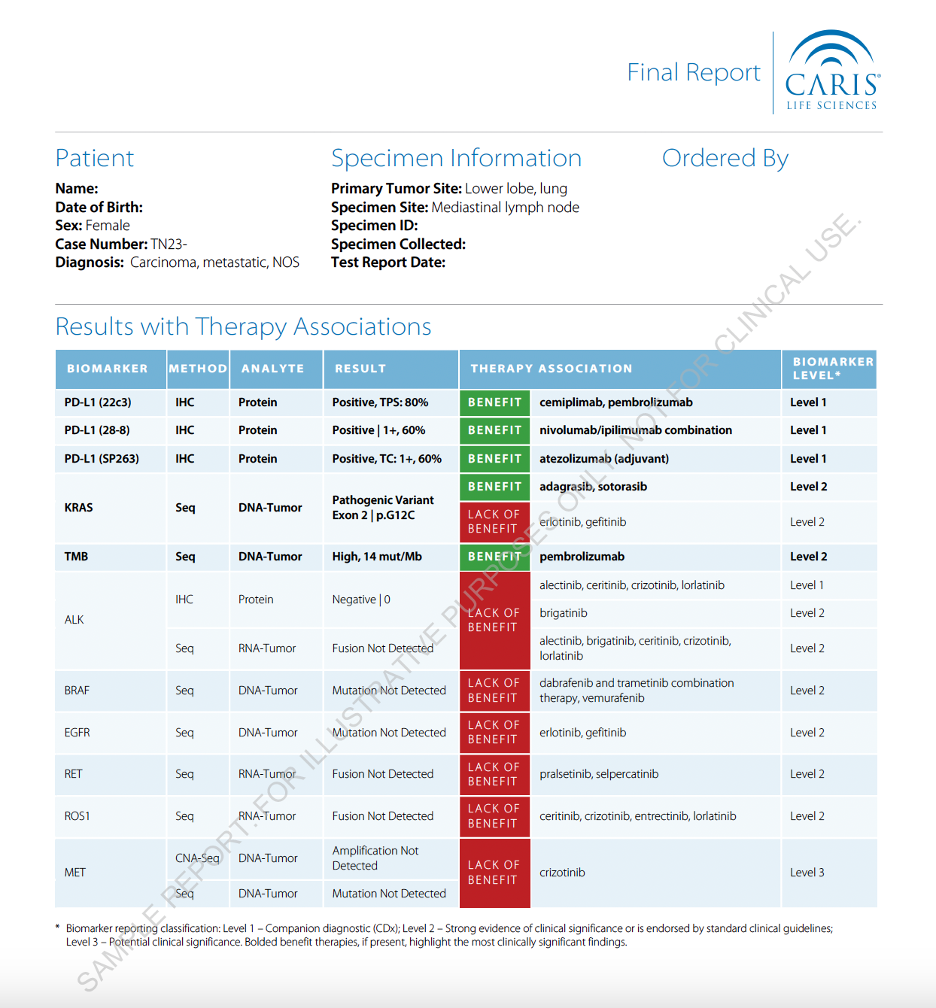
Complete Molecular Intelligence Report
The Caris Molecular Profiling Report delivers high impact results, including potentially relevant, actionable clinical information, in an easy-to-interpret format. Every report includes access to the MI Portal and the Clinical Trials Connector™, which matches each patient’s unique biomarker expression profile to open, pertinent, clinical trial opportunities.
Please complete the form below to have a Caris Scientific Rep (Medical Science Liaison) contact you directly.
"*" indicates required fields