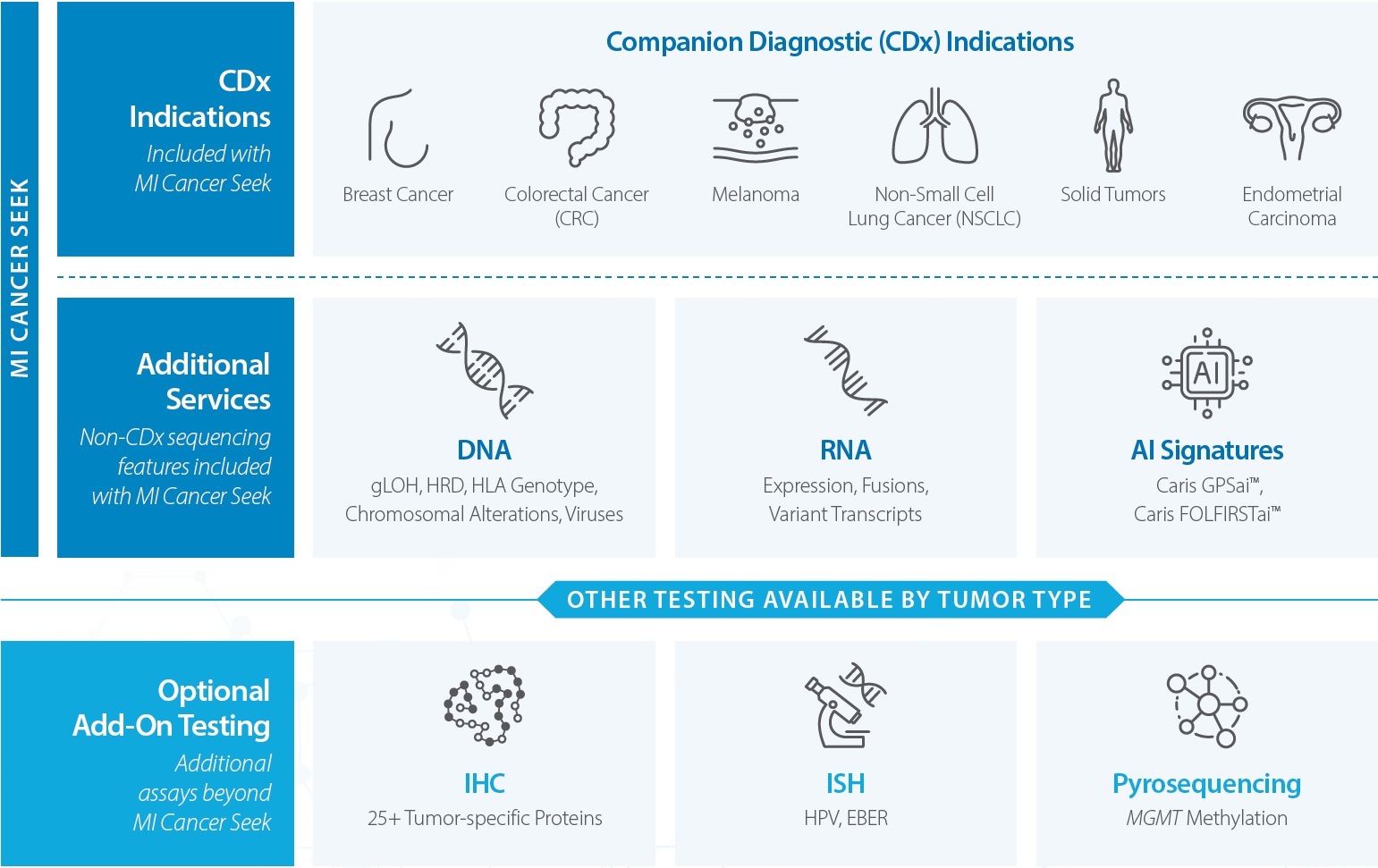
MI Cancer Seek
MI Cancer Seek® is the first and only simultaneous Whole Exome Sequencing (WES) and Whole Transcriptome Sequencing (WTS)-based assay with FDA-approved CDx indications for molecular profiling of solid tumors. MI Cancer Seek is available for adult and pediatric (ages 1-22) patients.
FDA-Approved CDx Indications and Tumor Profiling
MI Cancer Seek is a next-generation sequencing (NGS) based in vitro diagnostic device using total nucleic acid (TNA) isolated from formalin-fixed paraffin embedded (FFPE) tumor tissue specimens for the detection of SNVs, InDels, MSI, TMB in patients with previously diagnosed solid tumors, and CNA in one gene in patients with breast cancer. MI Cancer Seek is intended as a companion diagnostic to identify patients who may benefit from treatment with the targeted therapies listed in the Companion Diagnostic Indications table, in accordance with the approved therapeutic product labeling.
Additional Services
Beyond the MI Cancer Seek FDA-approved indications, additional sequencing-based features are reported.
- DNA: gLOH, HRD, HLA Genotype, Chromosomal Alterations, Viruses (HPV 16 & 18, EBV, MCPyV)
- RNA: Expression, Fusions, Variant Transcripts
- AI Signatures: Caris GPSai™, Caris FOLFIRSTai®
SPECIMEN TYPE(S)
Tissue (FFPE)
APPLICATION
Solid tumor profiling for therapy selection
MI Cancer Seek Companion Diagnostic Indications
Indication |
Biomarker |
Therapy |
Breast Cancer |
PIK3CA (C420R; E542K; E545A, E545D [1635G>T only], E545G, E545K, Q546E, Q546R; and H1047L, H1047R, H1047Y) |
PIQRAY® (alpelisib) |
Colorectal Cancer (CRC) |
KRAS wild-type (absence of mutations in exons 2, 3, and 4) and NRAS wild type (absence of mutations in exons 2, 3, and 4) |
VECTIBIX® (panitumumab) |
|
BRAF V600E |
BRAFTOVI® (encorafenib) in combination with ERBITUX® (cetuximab) |
Melanoma |
BRAF V600E |
BRAF inhibitors approved by FDA* |
|
BRAF V600E or V600K |
MEKINIST® (trametinib) or BRAF/MEK inhibitor combinations approved by FDA* |
Non-Small Cell Lung Cancer (NSCLC) |
EGFR exon 19 deletions and exon 21 L858R alterations |
EGFR Tyrosine Kinase Inhibitors approved by FDA* |
Solid Tumors |
MSI-H |
KEYTRUDA® (pembrolizumab), JEMPERLI® (dostarlimab-gxly) |
Endometrial Carcinoma |
Not MSI-H |
KEYTRUDA® (pembrolizumab) in combination with LENVIMA® (lenvatinib) |
*For the most current information about the device indications for the therapeutic products in this group, go to:
https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools#Group_Labeling
PIQRAY® is a registered trademark of Novartis AG. VECTIBIX® is a registered trademark of Immunex Corporation. BRAFTOVI® is a registered trademark of Array BioPharma Inc. in the United States and various other countries. ERBITUX® is a registered trademark of ImClone LLC, a wholly owned subsidiary of Eli Lilly and Company. MEKINIST® is a registered trademark of Novartis AG Corporation Switzerland. KEYTRUDA® is a registered trademark of Merck. JEMPERLI® (dostarlimab-gxly) is a registered trademark owned by the GSK group of companies. LENVIMA® (lenvatinib) is a registered trademark used by Eisai Inc. under license from Eisai R&D Management Co., Ltd.
MI Cancer Seek – Technical Specifications and Performance

TECHNOLOGY
Next-generation sequencing of total nucleic acids
Variant coverage
Somatic Tumor:
SNVs InDels CNAs
GENOMIC SIGNATURES
MSI TMB
MOLECULAR AI
Caris FOLFIRSTai (mCRC cases)
Caris GPSai (CUP cases)
SPECIMEN QUANTITY†
TNA extraction with ≥50 ng of DNA
(~10 slides at 20% tumor nuclei)
BREAST
PIK3CA
PPA: 99.4%
NPA: 100%
CRC
KRAS & NRAS
Wild-type
BRAF V600E
PPA: 100%
NPA: 97.2%
PPA: 100%
NPA: 97.2%
MELANOMA
BRAF V600E
BRAF V600E/K
PPA: 98.7%
NPA: 99.4%
PPA: 98.9%
NPA: 99.3%
NSCLC
EGFR exon 19 deletions and exon 21 L858R alterations
PPA: 98.1%
NPA: 99.4%
Solid Tumors
MSI-H
PPA: 97.5%
NPA: 98.5%
Endometrial
Not MSI-H
PPA: 98.4%
NPA: 97.6%
†TNA extraction with ≥50 ng of DNA is the minimum assay input requirement to perform MI Cancer Seek™. If insufficient quantity is submitted (<50 ng), the order for MI Cancer Seek will automatically reflex to MI Tumor Seek Hybrid™ (LDT).
Order Profiling
Email the completed form(s) to CustomerSupport@CarisLS.com, or fax to 1.866.479.4925. When specimen is being prepared for shipment, please include completed forms with the shipper.
Tour Our Tissue Lab
Intended Use
MI Cancer Seek is a next-generation sequencing (NGS) based in vitro diagnostic (IVD) device using total nucleic acid (TNA) isolated from formalin-fixed paraffin-embedded (FFPE) tumor tissue specimens for the detection of single nucleotide variants (SNVs) and insertions and deletions (indels) in 228 genes, microsatellite instability (MSI), tumor mutational burden (TMB) in patients with previously diagnosed solid tumors, and copy number amplification (CNA) in one gene in patients with breast cancer.
MI Cancer Seek is intended as a companion diagnostic to identify patients who may benefit from treatment with the targeted therapies listed in the Companion Diagnostic Indications table, in accordance with the approved therapeutic product labeling.
Additionally, MI Cancer Seek is intended to provide tumor mutational profiling to be used by qualified healthcare professionals in accordance with professional oncology guidelines for cancer patients with previously diagnosed solid malignant neoplasms. Genomic findings other than those listed in the Companion Diagnostic Indications table are not prescriptive or conclusive for labeled use of any specific therapeutic product.
Enhance Molecular Insights with Additional Services
MI Cancer Seek is a single assay that analyzes total nucleic acids for both DNA and RNA. Typically, DNA and RNA analysis by NGS requires two separate testing processes, which may require more tissue and more time. By combining WES and WTS into one assay, however, MI Cancer Seek provides a comprehensive molecular blueprint that saves tissue without compromising results.
Caris+Portal
Convenient Access to Caris Profiling
Caris+™Portal provides easy access for users to electronically submit orders, track case progress, view results and review Caris Life Sciences’ profiling information in one convenient location.
New users will select the Register link at the bottom of the login page and enter their name and their clinic or institution email address to verify the account.

Easy-to-Interpret Results
for Clarity in Treatment Planning
Treatment Planning:
- Navigate among FDA-approved drugs and therapies with potential benefit or lack of benefit
- Identify therapies that may not have been considered
- Match patient to clinical trials based on tumor biology
Evidence-guided:
- Drug associations based on peer-reviewed literature and clinical treatment guidelines
- Testing methodologies consistent with industry guidelines
EHR Compatible:
- Real-time sync
- HITECH compliant
- Easy installation
- Secure encryption
Technical Information
For complete product information, including companion diagnostic indications and performance characteristics, view the Technical Information document.
- For in vitro diagnostic use.
- CAUTION: Federal law restricts this device to sale by or on the order of a physician.
- The acceptable preparation method for MI Cancer Seek CDx specimens is FFPE. Other preparations have not been evaluated.
- The test is designed to report out somatic variants and is not intended to report germline variants.
- MI Cancer Seek requires a minimum tumor percentage of 20% for detection of alterations, with tumor content enrichment recommended for specimens with tumor percentage lower than 20%.
- Genomic findings other than those listed in the Companion Diagnostic Indications table are not prescriptive or conclusive for labeled use of any specific therapeutic product. Confirmation of tumor mutation status using an FDA-approved CDx test is needed for therapeutic use.
- A negative result does not rule out the presence of a mutation below the limits of detection of the assay.
- MI Cancer Seek is only approved for use with Caris Life Sciences pre-qualified Illumina NovaSeq 6000 instruments.
- The test is intended to be performed on specific serial number-controlled instruments by Caris Life Sciences.
- MI Cancer Seek does not report TMB for values lower than 3mut/Mb as the accuracy of TMB values are unreliable.
- Decisions on patient care and treatment must be based on the independent medical judgment of the treating physician, taking into consideration all applicable information concerning the patient’s condition, such as patient and family history, physical examinations, information from other diagnostic tests, and patient preferences, in accordance with the standard of care in a given community.
- Based on the low positive percent agreement (PPA) in the accuracy study for ERBB2 copy number amplifications (CNAs) in breast cancer patients, this alteration may not be detected. Additional clinical investigation to confirm the presence of ERBB2 CNAs in the breast cancer patient’s tumor with another FDA approved or cleared test is strongly recommended.
- Patients with breast cancer whose specimens have intermediate ERBB2 CNA status should be tested with another FDA approved or cleared test to ascertain ERBB2 CNA status in their tumor.
- Test may have reduced sensitivity and may yield false negative results in samples where necrotic tissue is >15%, melanin is >5%, fat cells are >10%.
Discover
More
Caris MI Profile® comprehensive testing delivers whole exome sequencing (WES – DNA) and whole transcriptome sequencing (WTS – RNA) for 23,000+ genes, as well as protein analysis and AI-predictive algorithms.
Caris partners with biopharma to provide multi-omic data that is fueling the next wave of biotherapeutics. Gain actionable insights and build tailored solutions for each phase of development.
Have Questions?
"*" indicates required fields